Description:
Fluorescent rocks absorb ultraviolet light and emit optical light, making them appear to glow in the dark.
Watch Video:
Other Demos of Interest:
Possible Incorporated Topics:
- light
- luminescence
- electron excitation
- absorption
- emission
Theory:
Fluorescence is when a substance absorbs light of a given wavelength and re-emits light of a longer wavelength. For example, a material that absorbs ultraviolet light and emits optical light is fluorescent. As the human eye cannot see ultraviolet light, fluorescent objects can appear to “glow in the dark”.
Wavelength and energy are inversely proportional, as the energy of a photon is given by:
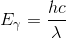
where h is Planck’s constant, c is the speed of light, and λ is the photon’s wavelength. A fluorescent material thus emits light of a lower energy than the light it absorbed. The material emits some energy in the form of heat to make up this difference in energy.
To understand fluorescence, let’s look at an electron energy level diagram:
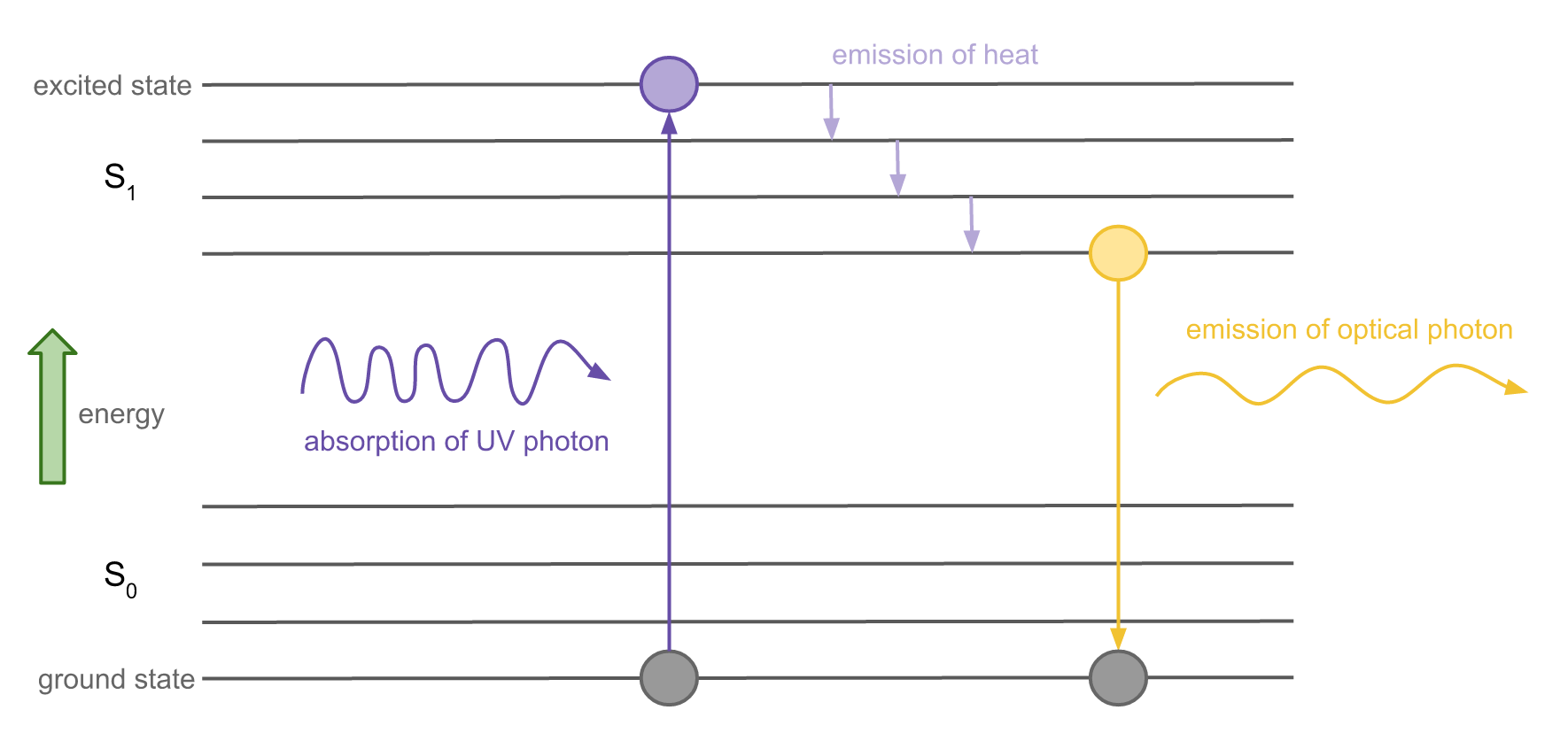
Each horizontal line represents a possible energy level of an electron with energy increasing vertically. The lowest possible state is the ground state (S0), while the highest states are excited states (S1). To jump between energy levels, the electron must gain or lose a certain amount of energy.
When an electron is hit by an incoming photon, the energy possessed by that photon can transfer to the electron and cause it to jump up to an excited state. This is called excitation.
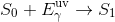
While the electron is excited, it releases some energy in the form of heat. Electrons prefer to be in the ground state so it will eventually jump back down, releasing energy during the jump in the form of a photon. Since it lost energy in the form of heat before jumping down, the jump back down releases a photon that less energy than the photon that was absorbed. The emitted photon is thus of a longer wavelength than the absorbed photon.
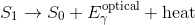
Fluorescence is a form of luminescence, which is when a substance emits light without being heated. Another form of luminescence is phosphorescence. Phosphorescent materials continue to emit light when they are no longer absorbing photons, unlike fluorescent materials which only glow under a lamp. The precise wavelength of the photons emitted depend on the material that is fluorescing. Thus, different materials will fluoresce different colours.
The four rocks featured in this video are fluorite (fluoresces blue), wernerite (fluoresces orange), willemite (fluoresces green), and manganapatite (fluoresces gold). They exhibit different colour fluoresces due to the different mineral compositions of each rock.

Apparatus:
- UV lamp
- fluorescent material (we used fluorescent rocks)
Procedure:
- turn off the lights and turn on the UV lamp
- place the fluorescent material beneath the lamp and watch it glow (emit optical photons)






